Blood Vessels
Section I: Blood Vessels and their parts
Introduction:
The tissues in our body are derived from the embryonic ectoderm, endoderm and mesoderm. The middle layer mesoderm provides supportive functions. This layer, which includes blood cells and blood vessels, form the supportive tissues of the body. The space between the ectoderm and endoderm is filled by the cavity the coelom that stretch from the neck to the posterior of the body. The linings of the body cavity from the mesoderm has changed throughout the evolution and development of chick mesoderm as shown in the figure and can be compared with the similar stages of frog embryo.
The blood throughout the human body is circulated by the blood vessels. These are major parts of the circulatory system and essential for transfer of important nutrient to various organs. The exchange of water and chemicals between blood and other tissues takes place in the capillaries. The other two major types of blood vessels are arteries and veins. Blood vessels are composed of two types of cells. One is lining the inner walls of the vessels, the endothelial cells, and other envelop the surface of the vascular tube the perivascular cells usually referred to as pericytes, these cells are also known as mural cells. The blood supply by the blood vessels depends on the endothelial cells. They have the capacity to adjust accordingly to the requirement of blood vessels.
Structure of Blood Vessels:
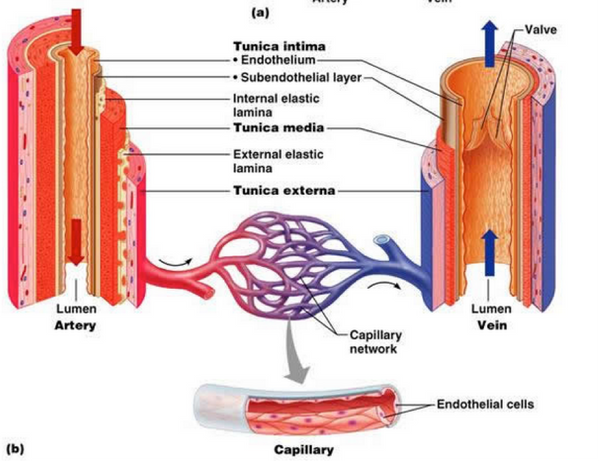
Blood vessels consist of arteries and veins, these are composed of three layers the middle layer of arteries is thicker than that of veins. Structurally these layers are as follow:
Tunica Intima: It is the thinnest layer having single layer of simple squamous endothelial type of cells, which are joined by polysaccharide matrix. This layer is surrounded by a thin layer connective tissue which are interconnected by number of circularly arranged elastic band known as internal elastic lamina.
Tunica Media: It is the thickest layer in the arteries and are circularly arranged elastic fibers, connective tissue. This layer separates the second and third layer by an elastic band known as external elastic lamina. This layer is rich in vascular smooth muscles in arteries and controls the caliber and quality of the vessels.
Tunica Adventitia: It is the thickest layer in veins and is made up of connective tissues. The nerves are located in this layer and in the larger blood vessels.
Formation of Blood Vessels:
The first functional organ of the body is heart and it requires circulatory loops to begin pumping the blood in the body. The blood vessels are formed independently and links to the heart soon after its formation. The genome of any individual cannot encode the intricate series of connections between the arteries and veins. All the circulatory systems look very much alike.
Constraints on Blood vessels formation:
There are many constraints on the development of vascular system. The embryonic cells need nourishment before the formation of intestines, need oxygen before the formation and lungs and similarly excrete wastes before the formation of kidney. These functions are mediated by the circulatory system. In an embryo the major blood vessel must be constructed to play a role as extra embryonic structures.
The evolutionary constraint on the blood vessels is that the embryo of mammals extends blood vessels to the yolk sac. The blood that leaves the heart moves through the vessels that forms a loop over the foregut and reach the dorsal aorta. There are six pairs of aortic arches that loop over the pharynx. The aortic arches of human embryo pumps the blood into the aorta and forms the branches on the sides of foregut. Another constraint on the blood vessels is the physical constraint according to the fluid dynamics. Only the larger tubes can effectively transport the blood. Blood vessels with smaller radius resist the increase in blood flow. The solution to this problem was the development of many smaller vessels. The construction of circulatory system negotiated these physical and evolutionary constraints.
Vasculogenesis:
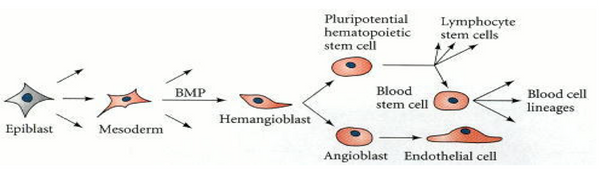
This is the process of formation of new blood vessels from the blood islands and both the blood cells and blood vessels comes from a common precursor the hemangioblast. These not only share common origin but any mutation to the transcription factors will affect both blood vessels and cells. The earliest blood cells and capillary have same rare proteins on their surfaces (Wood et al. 1997; Choi et al. 1998; Liao and Zon 1999).
The hemangioblasts cells are formed from the mesodermal cells and with high concentration of bone morphogenetic proteins. These are exposed during the early phase of development and form angioblasts.
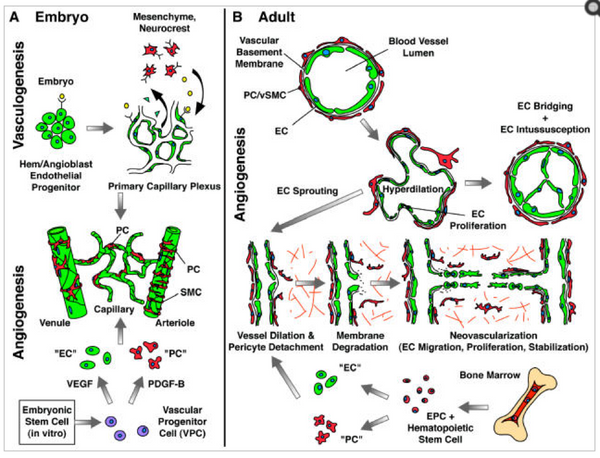
There are two processes of formation of blood vessesls: Vasculogenesis and angiogenesis.
In vasculogenesis the blood vessels are formed de novo from the lateral plate of mesoderm. In the first step the hemangioblasts cells are formed from the splanchnic mesodermal cells and these are the precursor of both the blood vessels and blood cells (Shalaby et al. 1997). These cells are often regarded as the blood islands. This blood islands have inner cells which form the hematopoietic stem cells which form blood cells and the outer cells of blood islands modify to angioblasts which are the precursors of blood vessels. In the second step of vasculogenesis, the endothelial cells which lines the inner walls of blood vessels are formed from the angioblasts. In the third step these newly formed endothelial cells form the tubes and develop a capillary plexus, which is a network of capillaries.
In angiogenesis the primary network of capillaries is reformed and pruned into capillary bed, arteries and veins (Risau 1997; Hanahan 1997). The capillary network of each organ is formed within that organ it does not comes from the larger vessels (Auerbach et al. 1985; Pardanaud et al. 1989).
Growth Factors for Vasculogenesis:
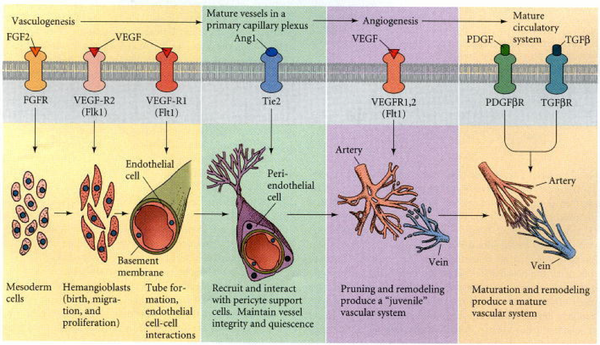
There are three growth factors which are responsible for vasculogenesis. These include fibroblast growth factor (FGF2) which is necessary for the generation of hemangioblasts from the splanchnic mesoderm. When the cells from quail blastodisc are cultured in FGF2, they form Blood Island and form endothelilal cells (Flamme and Risau 1992). Other growth factor include vascular endothelial growth factor (VEGF), this growth factor helps in differentiation of the angioblasts and multiply to form endothelial tubes. The third growth factor protein include angiopoietic-1 (Ang1), it facilitate the interaction between the pericytes and endothelial cells. Any mutation to these protein may cause deficient smooth muscles around the vessels (Davis et al. 1996; Suri et al. 1996; Vikkula et al. 1996).
The primary capillary network is remodeled after the initial vasculogenesis. During this phase arteries and veins are made and the process is known as angiogenesis. The protein VEGF acts on the capillaries and cause the loosening of the cell contact. This loosening of the cells cause the endothelial cells to move and sprout form this region and form new vessels. The new vessels can also be formed from the primary capillary network by dividing an existing vessels into two. The loosening also cause to form wider vessels. The platelet growth factor is important to enhance the mechanical flexibility by recruiting the pericyte cells to the capillaries (Lindahl et al. 1997).
The process of angiogenesis is the major way of formation of blood vessels. The capillary network is derived from the sprouting cells of aorta in the forelimb bud (Evans 1909; Feinberg 1991). The regions which form the organs of body secrete their angiogenesis factors and helps in the migration of endothelial cells to those areas. VEGF protein also promotes the migration of endothelial cells for the formation of blood vessels on the organs.
Angiogenesis is the only critical process for the formation of tissues including tumors as tumors grow only by the direct blood flow from the blood vessels. In this way they secrete angiogenesis factors. To prevent the formation of tumors, it is important to inhibit the growth of such factor via the use of medical applications and prevent the metastasis (Fidler and Ellis 1994).
Cells of Blood Vessels:
The blood vessels are made up of two types of cells; the endothelial cells and the pericytes.
Endothelial cells:
The walls of blood vessels are lined by the single sheet of endothelial cells the basal lamina separate the endothelial cells from the surrounding outer layers. The endothelial lining is present in every vessels and the capillaries only consist of endothelial cells for the exchange of materials between the blood and tissues. The location of the endothelium is critical in sensing any change in the hemodynamic forces and blood borne signals and it respond by releasing several vasoactive substances. The vascular hemostasis is maintained by the balance between the endothelium derived relaxing and contracting factors. Any misbalance to this can leads to predisposes the vasoconstriction, impaired coagulation, pre oxidation, thrombosis, platelet activation.
The vascular tone of blood vessels is maintained by the Nitric Oxide which is the relaxing factor in the blood vessel (Moncada S, 1993). It also opposes the actions of endothelium derived contracting factors like endothelin-1 and helps in keeping the vessels relaxed for the proper flow of blood. The endothelium is also the source of production of vasoconstrictor peptide ET-1. This peptide augments the actions of other peptides like angiotensin-II, serotonin and also take part in platelet activation and facilitates the prothrombic phenotype.
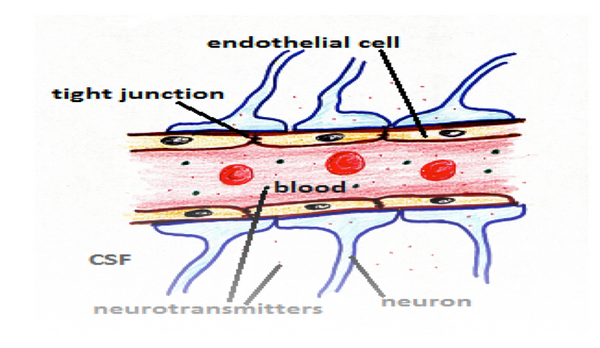
In the blood vessels the endothelial cells acts as a sieve and prevent the movement of large molecules, toxins and other microbes into the brain tissues and allow the flow of important molecules like oxygen, enzymes and hormones to the brain. With the flow of blood it helps in the removal of toxic waste like carbon dioxide out of the brain to waste disposal.
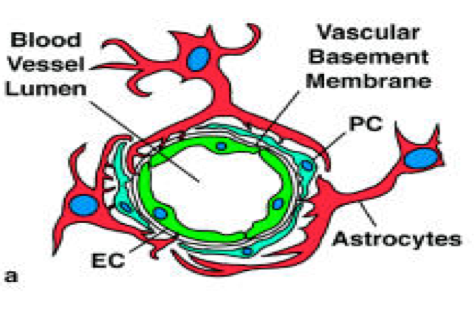
Pericytes:
The second type of cells present in the blood vessels are pericytes these are described as perivascular cells which are wrapped around the blood capillaries. These are formerly known as mural cells because of the contractile fibers and vascular smooth muscles present on them (Hirschi and D’Amore, 1996). The structural analysis via electron microscope revealed that pericyte possess a cell body having a nucleus and small amount of cytoplasm and long processes projecting into the endothelium wall. These are embedded into the basement membrane of micro-vessels (Mandarino et al., 1993).
Pericytes communicate with the endothelial cells by direct contact and via paracrine pathway. The plaques formed by the adhesion of pericytes to the endothelial cells enables it to penetrate in the vessel basement membrane and touch each other (Rucker et al., 2000). The long cytoplasmic processes of the pericytes extend longs and contact with more than one capillary in the vasculature. This cell-cell contact seems important for the activation of growth factors like TGF- β1, which promotes the differentiation of pericytes (Orlidge and D’Amore, 1987). The larger blood vessels are coated with multiple layers of smooth-muscle cells and elastic fibers (Cleaver and Melton, 2003). The pericytes cells in the blood vessels are associated with the stabilization and hemodynamic process of blood vessels. They sense the angiogenic stimuli and guide the proper sprouting of the tubes, facilitating the endothelial survival functions.
Pericytes in the Regulation of Blood Flow:
Like the smooth muscle cells in the larger vessels, pericytes also regulates the vascular diameter and capillary blood flow (Rucker et al., 2000). The contractile proteins like α-SMA, tropomyosin, and myosin present in the pericyte which perform these functions. The receptors for cholinergic and adrenergic receptors is also identified on the pericytes which regulate the blood flow. The vasoactive substances binds to the pericytes and perform the function of vessel relaxtion by a cGMP dependent mechanism. The oxygen level regulates the level of pericyte contraction. The in vivo contraction of pericyte is difficult to prove and the ultrastructure techniques shows the compression of endothelial cells by opposing pericytes. The contraction of pericytes was determined in respone to the vasoactive substances in skeletal muscles (Hirschi and D’Amore, 1996; Tilton et al., 1979).
Tissue specific Functions of Pericytes:
The density of pericyte differ with respect to the function of vessesl and organs where they are found. These are found abundantly in the small venules and arterioles. It has been seen they cover the endothelial cell junction most specifically during inflammation (Sims, 2000). The density of pericyte is dependent on blood pressure level and it is interesting to note that the level of pericyte is abundant down the torso and legs where there is increased pressure and the pumping of blood is in upward direction (Sims, 2000). The level of pericyte also differs among the tissues. This is due to the specialized characteristics in different organs and the function each organ perform. Pericyte perform specific functions in several organs like brain, liver and kidney.
Role in Vascular Development and Angiogenesis:
Pericytes plays an important role in the vessels formation. They have complex ontogeny and develop from various cells. It can develop from the neurocrest in the forebrain and cardiac tracts (Bergwerff et al., 1998; Etchevers et al., 2002). The best described origin of pericytes is from mesenchymal stem cells (Creazzo et al., 1998). It has been reported that pericytes are generated from endothelial transdifferentiation in a TGF-β3-dependent manner as seen in the dorsal aorta and cardiac valves (Gittenberger-de Groot et al., 1999) (Nakajima et al., 1997).
The vascular polarity and arteriovenous vessel specification shows that blood flow and pressure are the major determinants of arterial and venous vessel generation. The genetic mutation studies shows that VEGF genes in quail indicate that the fate of endothelial cells is predetermined before the circulation of blood is initiated (Carmeliet, 2004).
Conclusion:
The data described above shows that the blood vessels are produced from the blood island formed in the embryo and the cells of blood vessels are important in maintain the structure and function of the blood vessels. The endothelium and pericytes are the essential components of the micro-vessel walls and important in signaling and mechanical roles and support the endothelial cells and it depends on the angiogenic stage. Pericytes contributes to the regulation of pathological angiogenic processes. These findings leads to the new concept of antiangiogenic therapy and combined targeting of endothelial cells and pericytes.
References:
Wood H B, May G, Healy L, Enver T, Morriss-Kay G M. CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood. 1997;90:2300–2311.
http://www.ncbi.nlm.nih.gov/pubmed/9310481
Choi H, Kennedy M, Kazarov A, Padaimitriou J C, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732.
http://www.ncbi.nlm.nih.gov/pubmed/9435292
Shalaby F, 7 others. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990.
http://www.ncbi.nlm.nih.gov/pubmed/9200616
Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674.
http://www.ncbi.nlm.nih.gov/pubmed/9109485
Auerbach R, Alby L, Morrissey L, Tu M, Joseph J. Expression of organ-specific antigens on capillary endothelial cells. Microvasc. Res. 1985;29:401–411.
http://www.ncbi.nlm.nih.gov/pubmed/2582227
Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50.
http://www.ncbi.nlm.nih.gov/pubmed/9229772
Pardanaud L, Yassine F, Dieterlen-Lièvre F. Relationship between vasculogenesis, angiogenesis, and hemopoiesis during avian ontogeny. Development. 1989;105:473–485
http://www.ncbi.nlm.nih.gov/pubmed/2612361
Flamme I, Risau W. Induction of vasculogenesis and hematogenesis in vitro. Development. 1992;116:435–439.
http://www.ncbi.nlm.nih.gov/pubmed/1286617
Davis S, 10 others. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion trap expression cloning. Cell. 1996;87:1161–1169.
http://www.ncbi.nlm.nih.gov/pubmed/8980223
Suri C, 7 others. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180.
http://www.ncbi.nlm.nih.gov/pubmed/8980224
Vikkula M, 11 others. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181–1190.
http://www.ncbi.nlm.nih.gov/pubmed/8980225
Lindahl P, Johansson B R, Levéen P, Betscholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245.
http://www.ncbi.nlm.nih.gov/pubmed/9211853
Feinberg, R. N. 1991. Vascular development in the embryonic limb bud. In R. N. Feinberg, G. K. Sherer and R. Auerbach (eds.), The Development of the Vascular System. Issues in Biomedicine 14. Karger, Basel, pp. 136–148.
Evans H M. On the earliest blood vessels in the anterior limbs of birds and their relation to the primary subclavian artery. Am. J. Anat. 1909;9:281–319.
Fidler I J, Ellis L M. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;70:185–188.
http://www.ncbi.nlm.nih.gov/pubmed/7525076
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993; 329: 2002–2012
http://www.ncbi.nlm.nih.gov/pubmed/7504210
Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305.
http://www.ncbi.nlm.nih.gov/pubmed/10024303
Mandarino LJ, Sundarraj N, Finlayson J, Hassell HR. Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res. 1993;57:609–621.
http://www.ncbi.nlm.nih.gov/pubmed/8282048
Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462
http://www.ncbi.nlm.nih.gov/pubmed/3654761
Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull.2000;51:363–369.
http://www.ncbi.nlm.nih.gov/pubmed/10715555
Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668.
http://www.ncbi.nlm.nih.gov/pubmed/12778164
Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698
http://www.ncbi.nlm.nih.gov/pubmed/8915187
Tilton RG, Kilo C, Williamson JR. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc Res. 1979;18:325–335.
http://www.ncbi.nlm.nih.gov/pubmed/537510
Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846.
http://www.ncbi.nlm.nih.gov/pubmed/11022980
Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ Res. 1998;82:221–231
http://www.ncbi.nlm.nih.gov/pubmed/9468193
Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255:538–561.
http://www.ncbi.nlm.nih.gov/pubmed/15078497
Etchevers HC, Couly G, Le Douarin NM. Morphogenesis of the branchial vascular sector. Trends Cardiovasc Med. 2002;12:299–304.
http://www.ncbi.nlm.nih.gov/pubmed/12458092
Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–286.
http://www.ncbi.nlm.nih.gov/pubmed/9558464
Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: A role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309.
http://www.ncbi.nlm.nih.gov/pubmed/9215644
Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol.1999;19:1589–1594.
http://www.ncbi.nlm.nih.gov/pubmed/10397674
